1.Yasukawa Z, Inoue R, Ozeki M, Okubo T,
Takagi T, Honda A, Naito Y. Effect of repeated consumption of partially
hydrolyzed guar gum on fecal
characteristics and gut microbiota: a randomized, double-blind,
placebo-controlled, and parallel-group clinical trial. Nutrients. 2019 Sep
10;11(9):2170.
2.Ohashi Y, Sumitani K,
Tokunaga M, Ishihara N, Okubo T, Fujisawa T. Consumption of partially
hydrolysed guar gum stimulates Bifidobacteria and butyrate-producing bacteria
in the human large intestine. Beneficial microbes. 2015 Aug;6(4):451-5.
3.Kapoor MP, Koido M,
Kawaguchi M, Timm D, Ozeki M, Yamada M, Mitsuya T,
Okubo T. Lifestyle related changes with partially hydrolyzed guar gum dietary
fiber in healthy athlete individuals–A randomized, double-blind, crossover,
placebo-controlled gut microbiome clinical study. Journal of Functional Foods.
2020 Sep 1;72:104067.
4.Rosli D, Shahar S, Manaf ZA,
Lau HJ, Yusof NY, Haron MR,
Majid HA. Randomized controlled trial on the effect of partially hydrolyzed
guar gum supplementation on diarrhea frequency and gut microbiome count among
pelvic radiation patients. Journal of Parenteral and Enteral Nutrition. 2021
Feb;45(2):277-86.
5.Heini AF, Lara-Castro C, Schneider H,
Kirk KA, Considine RV, Weinsier RL.
Effect of hydrolyzed guar fiber on fasting and postprandial satiety and satiety
hormones: a double-blind, placebo-controlled trial during controlled weight
loss. International Journal of Obesity. 1998 Sep;22(9):906-9.
6.Van de Ven ML, Westerterp-Plantenga MS, Wouters L,
Saris WH. Effects of liquid preloads with different fructose/fibre
concentrations of subsequent food intake and ratings of hunger in women.
Appetite. 1994 Oct 1;23(2):139-46.
7.Lluch A, Hanet-Geisen N,
Salah S, Salas-Salvadó J, L’Heureux-Bouron D,
Halford JC. Short-term appetite-reducing effects of a low-fat dairy product
enriched with protein and fibre. Food quality and preference. 2010 Jun
1;21(4):402-9.
8.Rao TP, Hayakawa M, Minami T, Ishihara N,
Kapoor MP, Ohkubo T, Juneja LR, Wakabayashi K. Post-meal perceivable satiety
and subsequent energy intake with intake of partially hydrolysed guar gum.
British Journal of Nutrition. 2015 May;113(9):1489-98.
9.Nakamura S, Hongo R,
Moji K, Oku T. Suppressive effect of partially hydrolyzed guar gum on
transitory diarrhea induced by ingestion of maltitol and lactitol in healthy
humans. European journal of clinical nutrition. 2007 Sep;61(9):1086-93.
10.Inoue R, Sakaue Y,
Kawada Y, Tamaki R, Yasukawa Z, Ozeki M, Ueba S, Sawai C, Nonomura K,
Tsukahara T, Naito Y. Dietary supplementation with partially hydrolyzed guar
gum helps improve constipation and gut dysbiosis symptoms and behavioral
irritability in children with autism spectrum disorder. Journal of clinical
biochemistry and nutrition. 2019;64(3):217-23.
11.Ustundag G, Kuloglu Z, Kirbas N,
Kansu A. Can partially hydrolyzed guar gum be an alternative to lactulose in
treatment of childhood constipation. Turk J Gastroenterol. 2010 Dec
1;21(4):360-4.
12.Kapoor MP, Ishihara N, Okubo T. Soluble
dietary fibre partially hydrolysed guar gum markedly impacts on postprandial
hyperglycaemia, hyperlipidaemia and incretins metabolic hormones over time in
healthy and glucose intolerant subjects. Journal of Functional Foods. 2016 Jun
1;24:207-20.
13.Trinidad T, Perez E, Loyola A, Mallillin A, Encabo R, Yokawa T,
Aoyama N, Juneja L. Glycemic index of Sunfibre (Cyamoposis
tetragonolobus) products in normal and diabetic subjects. International journal
of food science & technology. 2004 Dec;39(10):1093-8.
14.Quigley EM. Gut bacteria in health and
disease. Gastroenterology & hepatology. 2013 Sep;9(9):560.
15.Yoon SJ, Chu DC, Juneja LR. Chemical and
physical properties, safety and application of partially hydrolized guar
gum as dietary fiber. Journal of Clinical Biochemistry and Nutrition.
2008;42(1):1-7.
16.Yasukawa Z, Inoue R, Ozeki M, Okubo T,
Takagi T, Honda A, Naito Y. Effect of repeated consumption of partially
hydrolyzed guar gum on fecal
characteristics and gut microbiota: a randomized, double-blind,
placebo-controlled, and parallel-group clinical trial. Nutrients. 2019 Sep
10;11(9):2170.
17.Rosli D, Shahar S, Manaf ZA,
Lau HJ, Yusof NY, Haron MR,
Majid HA. Randomized controlled trial on the effect of partially hydrolyzed
guar gum supplementation on diarrhea frequency and gut microbiome count among
pelvic radiation patients. Journal of Parenteral and
18.Kapoor MP, Koido M,
Kawaguchi M, Timm D, Ozeki M, Yamada M, Mitsuya T,
Okubo T. Lifestyle related changes with partially hydrolyzed guar gum dietary
fiber in healthy athlete individuals–A randomized, double-blind, crossover,
placebo-controlled gut microbiome clinical study. Journal of Functional Foods.
2020 Sep 1;72:104067.
19.Majeed M, Nagabhushanam K,
Natarajan S, Sivakumar A, Pande A, Majeed S, Ali F. A double-blind,
placebo-controlled, parallel study evaluating the safety of Bacillus coagulans MTCC
5856 in healthy individuals. J Clin Toxicol.
2016;6(283):2161-0495.
20.Minamida K, Nishimura M, Miwa K, Nishihira J.
Effects of dietary fiber with Bacillus coagulans
lilac-01 on bowel movement and fecal properties of healthy volunteers with a
tendency for constipation. Bioscience, Biotechnology, and Biochemistry. 2015
Feb 1;79(2):300-6.
21.Majeed MU, Natarajan S, Sivakumar A, Ali
F, Pande AN, Majeed S, Karri SK. Evaluation of anti-diarrhoeal activity of
Bacillus coagulans MTCC
5856 and its effect on gastrointestinal motility in Wistar rats. International
Journal of Pharma and Bio Sciences. 2016;7(1):P311â.
22.Zhou Q, Verne ML, Fields JZ, Lefante JJ,
Basra S, Salameh H, Verne GN. Randomised placebo-controlled trial of dietary
glutamine supplements for postinfectious irritable bowel syndrome. Gut. 2019
Jun 1;68(6):996-1002.
23.Cresci GA, Glueck B, McMullen MR, Xin W, Allende D, Nagy
LE. Prophylactic tributyrin treatment mitigates chronic‐binge ethanol‐induced
intestinal barrier and liver injury. Journal of Gastroenterology and
Hepatology. 2017 Sep;32(9):1587-97.
24.Glueck B, Han Y, Cresci GA.
Tributyrin supplementation protects immune responses and vasculature and
reduces oxidative stress in the proximal colon of mice exposed to chronic-binge
ethanol feeding. Journal of Immunology Research. 2018 Oct;2018.
25.Miragoli F, Patrone V,
Prandini A, Sigolo S, Dell’Anno M,
Rossi L, Senizza A,
Morelli L, Callegari ML.
Implications of tributyrin on gut microbiota shifts related to performances of
weaning piglets. Microorganisms. 2021 Mar 12;9(3):584.
26.Inoue R, Sakaue Y,
Kawada Y, Tamaki R, Yasukawa Z, Ozeki M, Ueba S, Sawai C, Nonomura K,
Tsukahara T, Naito Y. Dietary supplementation with partially hydrolyzed guar
gum helps improve constipation and gut dysbiosis symptoms and behavioral
irritability in children with autism spectrum disorder. Journal of clinical
biochemistry and nutrition. 2019;64(3):217-23.
27.Rastgoo S,
Ebrahimi-Daryani N, Agah S,
Karimi S, Taher M, Rashidkhani B,
Hejazi E, Mohseni F, Ahmadzadeh M,
Sadeghi A, Hekmatdoost A.
Glutamine supplementation enhances the effects of a low FODMAP diet in
irritable bowel syndrome management. Frontiers in Nutrition. 2021:1079.
28.Balliett M, Burke JR. Changes in
anthropometric measurements, body composition, blood pressure, lipid profile,
and testosterone in patients participating in a low-energy dietary
intervention. Journal of chiropractic medicine. 2013 Mar 1;12(1):3-14.
29.Van de Ven ML, Westerterp-Plantenga MS, Wouters L,
Saris WH. Effects of liquid preloads with different fructose/fibre
concentrations of subsequent food intake and ratings of hunger in women.
Appetite. 1994 Oct 1;23(2):139-46.
30.Lluch A, Hanet-Geisen N,
Salah S, Salas-Salvadó J, L’Heureux-Bouron D,
Halford JC. Short-term appetite-reducing effects of a low-fat dairy product
enriched with protein and fibre. Food quality and preference. 2010 Jun
1;21(4):402-9.
31.Balliett M, Burke JR. Changes in
anthropometric measurements, body composition, blood pressure, lipid profile,
and testosterone in patients participating in a low-energy dietary
intervention. Journal of chiropractic medicine. 2013 Mar 1;12(1):3-14.
32.Majeed M, Nagabhushanam K,
Arumugam S, Majeed S, Ali F. Bacillus coagulans MTCC
5856 for the management of major depression with irritable bowel syndrome: a
randomised, double-blind, placebo controlled, multi-centre, pilot clinical
study. Food & nutrition research. 2018;62.
33.Grosicki GJ,
Riemann BL, Flatt AA, Valentino T, Lustgarten MS.
Self-reported sleep quality is associated with gut microbiome composition in
young, healthy individuals: a pilot study. Sleep medicine. 2020 Sep
1;73:76-81.
34.Sakai S, Kamada Y,
Takano H, Ichikawa M, Kurimoto M, Katsuyama HK, Nishihira J,
Sasaki M. Continuous partially hydrolyzed guar gum intake reduces cold-like
symptoms: a randomized, placebo-controlled, double-blinded trial in healthy
adults. European Review for Medical and Pharmacological Sciences. 2022 Jan
1;26(14):5154-63.
35.Cengiz M, Uysal BB, Ikitimur H, Ozcan E, Islamoğlu MS, Aktepe E, Yavuzer H, Yavuzer S.
Effect of oral l-Glutamine supplementation on Covid-19 treatment. Clinical
nutrition experimental. 2020 Oct 1;33:24-31.
36.Shinde T, Vemuri R, Shastri MD, Perera
AP, Tristram S, Stanley R, Eri R. Probiotic Bacillus coagulans MTCC
5856 spores exhibit excellent in-vitro functional efficacy in simulated gastric
survival, mucosal adhesion and immunomodulation. Journal of Functional Foods.
2019 Jan 1;52:100-8.
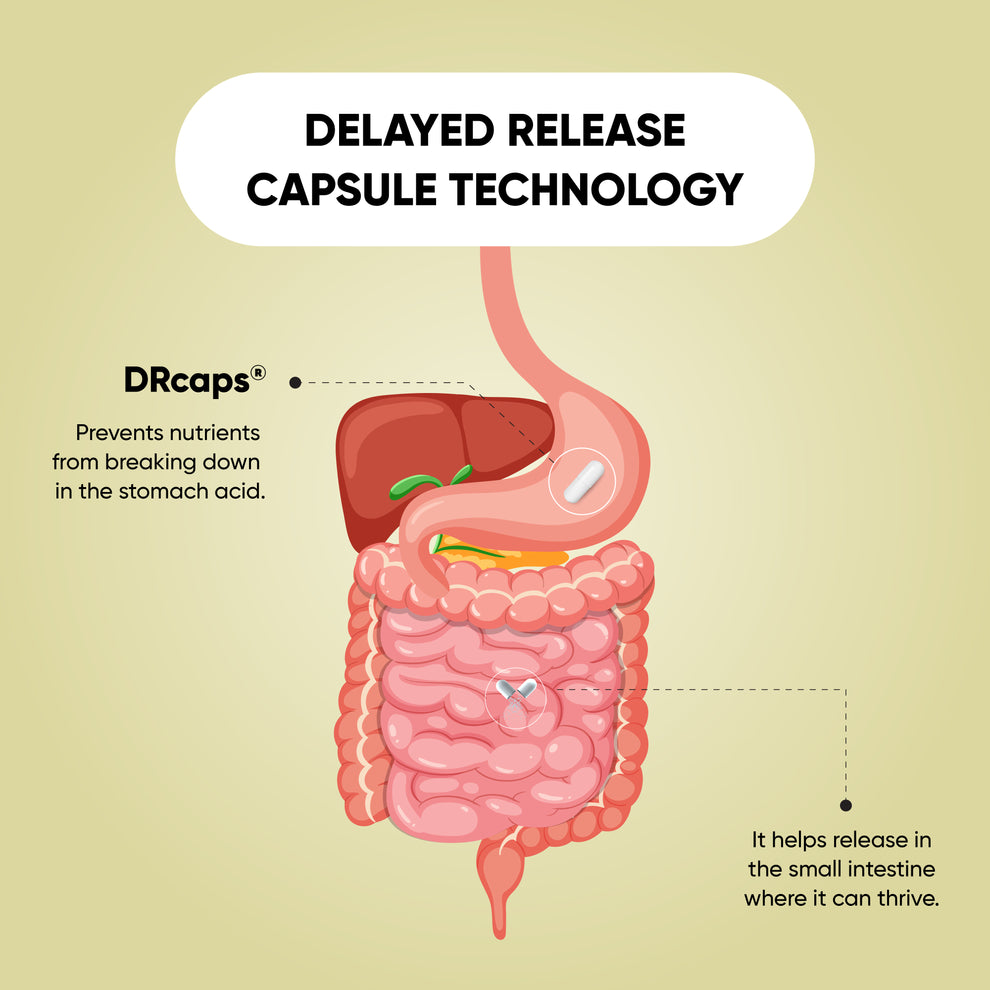
37.Amo R. DRcaps® capsules achieve delayed
release properties for nutritional ingredients in human clinical study. No.
BAS. 2014;420.